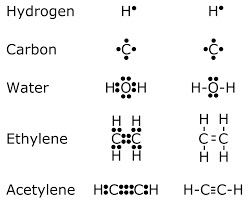
 |
| Examples of Lewis
dot-style representations of chemical bonds
between carbon (C), hydrogen (H), and oxygen
(O). Lewis dot diagrams were an early attempt to
describe chemical bonding and are still widely
used today. |
Bonding
A chemical bond is a lasting attraction between atoms,
ions or molecules that enables the formation of chemical
compounds. The bond may result from the electrostatic
force of attraction between oppositely charged ions as
in ionic bonds or through the sharing of electrons as in
covalent bonds. The strength of chemical bonds varies
considerably; there are "strong bonds" or "primary
bonds" such as covalent, ionic and metallic bonds, and
"weak bonds" or "secondary bonds" such as dipole–dipole
interactions, the London dispersion force and hydrogen
bonding.
Since opposite charges attract via a simple
electromagnetic force, the negatively charged electrons
that are orbiting the nucleus and the positively charged
protons in the nucleus attract each other. An electron
positioned between two nuclei will be attracted to both
of them, and the nuclei will be attracted toward
electrons in this position. This attraction constitutes
the chemical bond. Due to the matter wave nature of
electrons and their smaller mass, they must occupy a
much larger amount of volume compared with the nuclei,
and this volume occupied by the electrons keeps the
atomic nuclei in a bond relatively far apart, as
compared with the size of the nuclei themselves.
In general, strong chemical bonding is associated with
the sharing or transfer of electrons between the
participating atoms. The atoms in molecules, crystals,
metals and diatomic gases—indeed most of the physical
environment around us—are held together by chemical
bonds, which dictate the structure and the bulk
properties of matter. |
|